- Introduction
The EOR method in the form of gas injection which is known and proven to be successful in increasing oil recovery is gas injection using carbon dioxide (CO2) gas. This method is known as the CO2-EOR method which has been shown to increase oil recovery very significantly as happened in the Sacroc Unit, Dollarhide, Bell Creek, and Camurlu Fields (Gill, 1982; Bellavance, 1996; Gorecki et al., 2012; Gondiken, 1987).
It is known that in order to increase oil recovery, the CO2-EOR method can be applied to both heavy and light oils. Miscible CO2 injection has been shown to provide gains in the range of 10-20% of the original oil in-place (OOIP) content. If the reservoir pressure has been reduced then the injection of immiscible CO2 can be carried out. This method has been shown to significantly increase oil recovery in the range of 5-10% of the initial oil content (Lake, 1989).
- Problems in the field
Oil fields that have been produced through the primary stage for a long period of time without taking pressure maintenance measures will result in reservoir pressure dropping to very low levels. If this situation is not addressed, the oil production from the field will decrease to a very low level. In such circumstances, efforts should be made so that oil production can be increased again. According to the 2015 SKK Migas Report, the primary and secondary methods still leave a very significant amount of hydrocarbons in many oil fields in Indonesia. In fact, various efforts have been made to maintain the current primary production flow rate. However, the rate of decline in production cannot be maintained significantly so that further steps are needed, namely applying the advanced recovery method to increase oil recovery from mature fields or if reservoir pressure is depleted according to the potential required.
- Current solutions and proposed solutions
The short-term solution in an effort to increase or at least maintain the rate of oil production is to carry out various ways including stimulating wells, creating insertion wells, injection of water to maintain pressure, re-perforating, changing artificial lifting methods, and so on. . These efforts in the end were unable to continue to maintain production so that further methods of increasing yields were needed.
One of the further methods to increase the acquisition or enhanced oil recovery (EOR) which has been proven to increase oil production is gas injection using CO2 gas. The presence of CO2 gas in the air is very dominant and has been shown to make a major contribution to global warming (Howard et al, 2000). CO2 gas can be produced from oil and gas production activities. CO2 gas can also be produced from industrial activities such as cement factories, petrochemical industries, steel factories, coal power plants, and others (Dipietro, 2012). Besides being able to provide increased oil recovery, injection of CO2 gas into the oil reservoir can also help reduce the release of CO2 gas into the air. Thus, CO2 gas injection provides its own added value in environmental conservation efforts. This activity is known as CO2 sequestration and utilization which has been widely carried out in various developed countries.
2. Materials & Methods
This study was conducted by conducting experiments in the laboratory using standard equipment to determine the interfacial tension of a two-fluid system which in this case is a system consisting of CO2 gas and oil. The interfacial tension in this study was determined by calculation using the pendant drop method using goniometer equipment which has been equipped with a special view cell for high pressure and temperature conditions. The CO2 gas used has a purity level of 99.99%. The oil samples used came from the Air Benakat Formation in the South Sumatra Basin. The oil sample has an API gravity of 41.38 with the physical properties and composition as shown in Tables 1 and 2. The pressures are given from 700 psi to 1800 psi with three temperature values, namely 40 oC, 60 oC, and 80 oC. The pressure and temperature values are taken to describe the actual reservoir conditions.

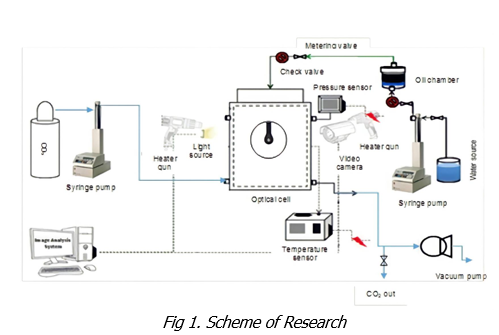
3. Result & Discussion
Pressure is proven to have a very significant effect on the decrease in interfacial tension between CO2 and oil. The experimental data in this study show that the increase in applied pressure can drastically reduce the interfacial tension between CO2 and oil, especially at low temperatures. In common sense, an increase in pressure causes the density of CO2 to increase and if the pressure increase continues, the price of CO2 density can approach the price of oil density. In other words, the density difference between CO2 and oil is getting smaller. When the density difference between the two fluids in contact is small, the interfacial tension between the two fluids in contact is relatively lower.
Temperature has the opposite effect on the interfacial tension of CO2 and oil where an increase in temperature results in an increase in interfacial tension between CO2 and oil. It is understood that the increase in temperature makes it more difficult for CO2 to dissolve in the oil. In addition, the increase in temperature causes a decrease in the density of CO2 at the same pressure. In other words, higher pressure is required to increase the CO2 density at higher temperatures. At the pressure and temperature used in the experiments in this study, it can be seen that the greater the difference in density between CO2 and oil, the greater the interfacial tension. Thus, an increase in temperature will result in a higher interfacial tension between CO2 and oil.
Further observation of the amount of interfacial tension at the three temperature values used shows that the interfacial tension at 40 oC decreases until it approaches zero. This indicates that at low temperatures the miscible or near-miscible mechanism is easier to occur even at relatively lower pressures. While the interfacial tension at the other two higher temperatures, namely at 60 oC and 80 oC, the interfacial tension can approach zero at a much higher pressure. Thus, at the maximum pressure value given in this experiment, the mechanism that occurs is immiscible.
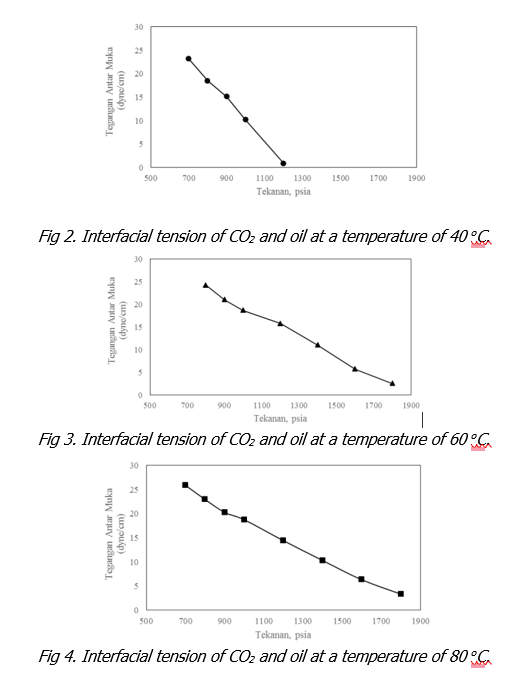
Furthermore, through visual observation, information was obtained about the effect of pressure and temperature on the size of the oil droplets hanging on the tip of the needle. From these observations, it can be seen that the increase in pressure causes the size of the oil droplets to be smaller. The change in droplet volume was mainly caused by the extraction process of the lighter components from the oil sample used. A further increase in pressure will result in a state in which only the heavy component remains in the droplet. If this situation continues, it will approach a mixed condition (miscible) or a nearly miscible condition (near miscible).
4. Conclusion
- In reservoir conditions, the interfacial tension of CO2-oil is strongly influenced by the amount of pressure and temperature.
- The increase in pressure causes a decrease in the CO2-oil interfacial tension. On the other hand, an increase in temperature can cause an increase in the CO2-oil interfacial tension.
- The increase in pressure causes the density difference between CO2 and oil to be smaller. This mechanism can cause a decrease in the CO2-oil interfacial tension.
- The increase in temperature causes the density difference between CO2 and oil to become larger. This phenomenon can cause the reduction of the CO2-oil interfacial tension to be more difficult at higher temperatures.The miscible mechanism between CO2 and oil, which is characterized by an interfacial tension close to zero, is more likely to occur at lower temperatures. Meanwhile, at higher temperatures, a much higher pressure is required so that the interfacial tension approaches zero. In conditions where the interfacial tension is greater than zero, the mechanism that occurs is immiscible.

No Responses